Calcite Crystals
| Chemical Formula: | CaCO3 |
| Color: | colorless, white, pink, yellow, or brown. |
| Diaphaniety: | transparent to translucent to opaque |
| Hardness: | 3 - Calcite |
| Density: | 2,72 |
| Luster: | vitreous (glassy) |
| Streak: | white |
| Crystal System: | Trigonal - Hexagonal Scalenohedral H-M Symbol (-3 2/m) Space Group: R3_c |
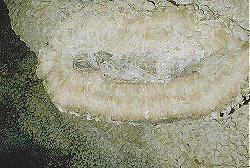
Calcite crystals are the most common crystals of limestone or calcium carbonate (CaCO3) in its mineral form. There are two possible forms of calcium carbonate, calcite and aragonite. Both have the same chemical formula, but different mineral structure. Calcite was named as a mineral by Gaius Plinius Secundus (Pliny the elder) in 79 from Calx, Latin for Lime.
In karst caves, and in many cases even in other caves, most formations, such as stalactites or stalagmites, consist of calcite crystals. These are very difficult to see on the surface, but quickly become clear at the fracture. This is also the reason why stalactites are usually translucent. The transparency is disturbed by air bubbles and also by clay minerals or other impurities. Dripstones are transparent like calcite crystals or if they contain air bubbles white but translucent. The colouration is usually caused by iron oxides, in the spectrum beige, brown, reddish or red.
The lime comes from the rock above the cave through which the drip water seeps. So it is quite common for dripstones to grow even in non-karst caves if there are deposits of limestone above. All other minerals are very rare in caves.
In contrast to calcite-based dripstones, calcite crystals are relatively rare in caves. They only form under water from supersaturated solution, which is quite normal for drip water. It is rare, however, for water to remain stagnant for very long periods without disturbance. Caves tend to have dynamic water courses, they represent the underground drainage of the karst area. However, crystals do not form in flowing water. When the drainage moves deeper, the cave falls dry and there is no more water. Only when an impermeable layer, usually cave clay, prevents water from draining away, forming a cave lake, and dripping water supplies the lake constantly with calcareous water, crystals can form. The longer the condition persists, the larger the crystals become. In very rare cases, such as Jewel Cave in the U.S.A., the cave is completely filled with stagnant water and the walls become completely covered with crystals.
 Search DuckDuckGo for "Calcite"
Search DuckDuckGo for "Calcite" Calcite - Wikipedia (visited: 19-AUG-2022)
Calcite - Wikipedia (visited: 19-AUG-2022) Calcite (visited: 19-AUG-2022)
Calcite (visited: 19-AUG-2022)
 Index
Index Topics
Topics Hierarchical
Hierarchical Countries
Countries Maps
Maps